Volume 83, Issue 23 7,December 2023
Xiaohan Wang1 ,Liangwen Ma1,2 ,Ningning Li,1,2,*,Ning Gao1,2,3,4,*

Cover design process
This cover is designed with a clear idea, and the process of DNA replication of monkeypox virus DNA polymerase F8 with the help of viral factors A22, E4 and H5 is cleverly transformed into a visual image. The two spirally entwined vines represent the template DNA strand and the nascent DNA strand of the viral genome respectively, which graphically demonstrates the transmission of genetic information during the DNA replication process.
The designers have used vivid colour combinations to transform the biomolecular mechanism into visual art. The template DNA strand is presented as a dark-coloured vine, while the nascent DNA strand is light-coloured, making the distinction between the two more obvious. At the same time, the use of various colours makes the whole design look more vivid and lively, which enhances readers' interest in reading.
In addition, the choice of background colour for the cover is also unique. The light green background makes the whole design more fresh and natural and complements the theme. This colour choice not only highlights the theme, but also makes the whole design look more harmonious and unified.
Overall, the cover design of Molecular Cell is certainly a successful attempt. It reveals the mysterious process of DNA replication of the monkeypox virus with a unique perspective and vivid graphic language, and at the same time, it shows the ingenious ideas and exquisite skills of the designer. This design not only conveys scientific knowledge, but also provides a novel artistic enjoyment. It successfully combines science and art, allowing us to have a deeper understanding of biomedical research while enjoying it. Eventually the cover was highly recognised by the teachers and journal editors and was successfully published!
The cover of this issue of Molecular Cell is the article“Structural insights into the assembly and mechanism of mpox virus DNA polymerase complex F8- A22-E4-H5”by Professor Ning Gao and Associate Researcher Ningning Li from Peking University.
Since the outbreak in May 2022, monkeypox virus has spread rapidly worldwide, posing a serious threat to human health. The monkeypox virus of the outbreak belongs to a large, enveloped, double-stranded DNA virus in the genus Orthopoxvirus of the family Poxviridae. In addition to this, the family also includes two well known members, vaccinia virus and variola virus. Important targets for the action of anti-orthopoxvirus drugs are the DNA replication machinery, in particular the DNA polymerase. The resolution of high-resolution structures is essential for the development of anti-monkeypox drugs and vaccines.
While most DNA viruses replicate their genomes in the nucleus of the host cell, genome replication of poxviruses is done in the host cytoplasm. Key protein factors required for monkeypox virus replication are mainly encoded by its genome, including DNA polymerase F8 (named after the monkeypox virus system), DNA helicases/primase E5, uracil-DNA glycosylase (UDG) E4, heterodimer A22, which together with E4 forms the polymerase replication persistence factor (processivity factor), and the multifunctional phosphorylated protein H5, among others.
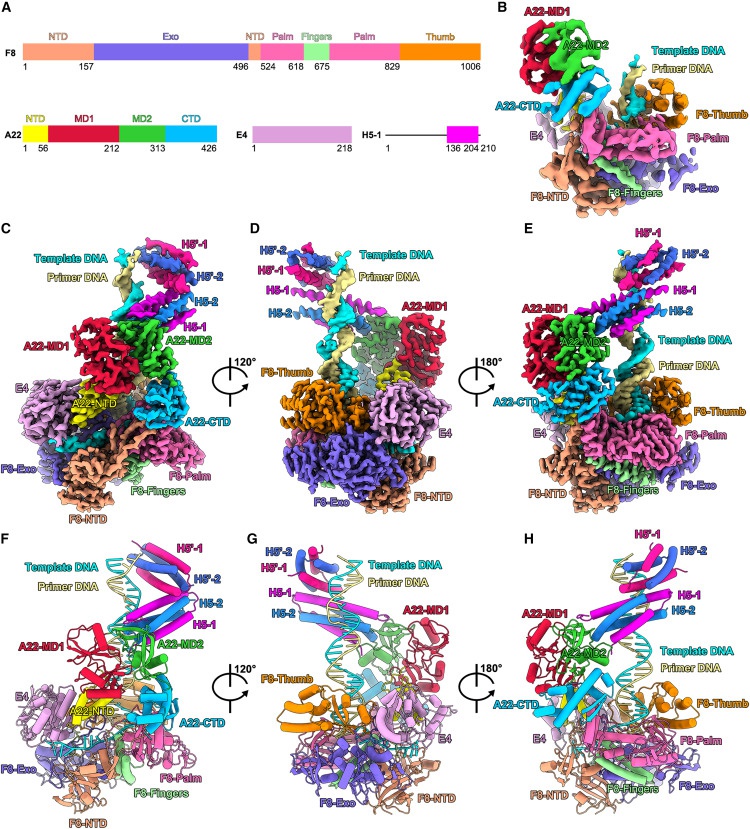
F8 is the core catalytic subunit of the DNA polymerase complex, but can only synthesise small fragment products of less than 10 bases.The A22-E4 dimer forms a stable ternary complex with F8, which enhances the persistence of polymerase replication, and thus the ternary complex is also considered to be the poxviral DNA polymerase holoenzyme. In addition, UDG E4 recognises and excises uracil single base mutations in DNA and initiates the DNA repair pathway. However, the mechanism by which UDG is integrated into the polymerase holoenzyme as an essential factor and the coupling mechanism between the two processes of DNA synthesis and uracil base search/excision remain unclear. Meanwhile, H5 was shown to be a multifunctional protein and an essential factor for poxvirus DNA replication, but the specific role of its molecular mechanism in the DNA replication process remains completely unknown.
In this study, the molecular mechanism of H5 tetramer-enhanced polymerase persistence was revealed by high-resolution F8-A22-E4-H5 structure and function experiments, which demonstrated that E4 has a base excision catalytic activity in the polymerase complex. Previous studies have shown that the poxvirus polymerase does not possess transdamage synthetic activity, which implies that the base-free site created by E4 after excision of the U base on the single-stranded DNA template requires further repair. Otherwise, this would affect DNA synthesis mediated by F8 in the quaternary complex, implying that the intact viral replicon may also include additional factors of the base excision repair pathway.
Overall, this work, together with related studies recently published by other teams, provides important structural and functional information about the unique DNA replication molecular mechanism of poxviruses. These findings contribute to a deeper understanding of the life cycle and replication strategy of poxviruses and provide new targets for antiviral drug development.
Our hours
Beijing time: 9:00-18:00